Learn how remanufacturing works
Overview
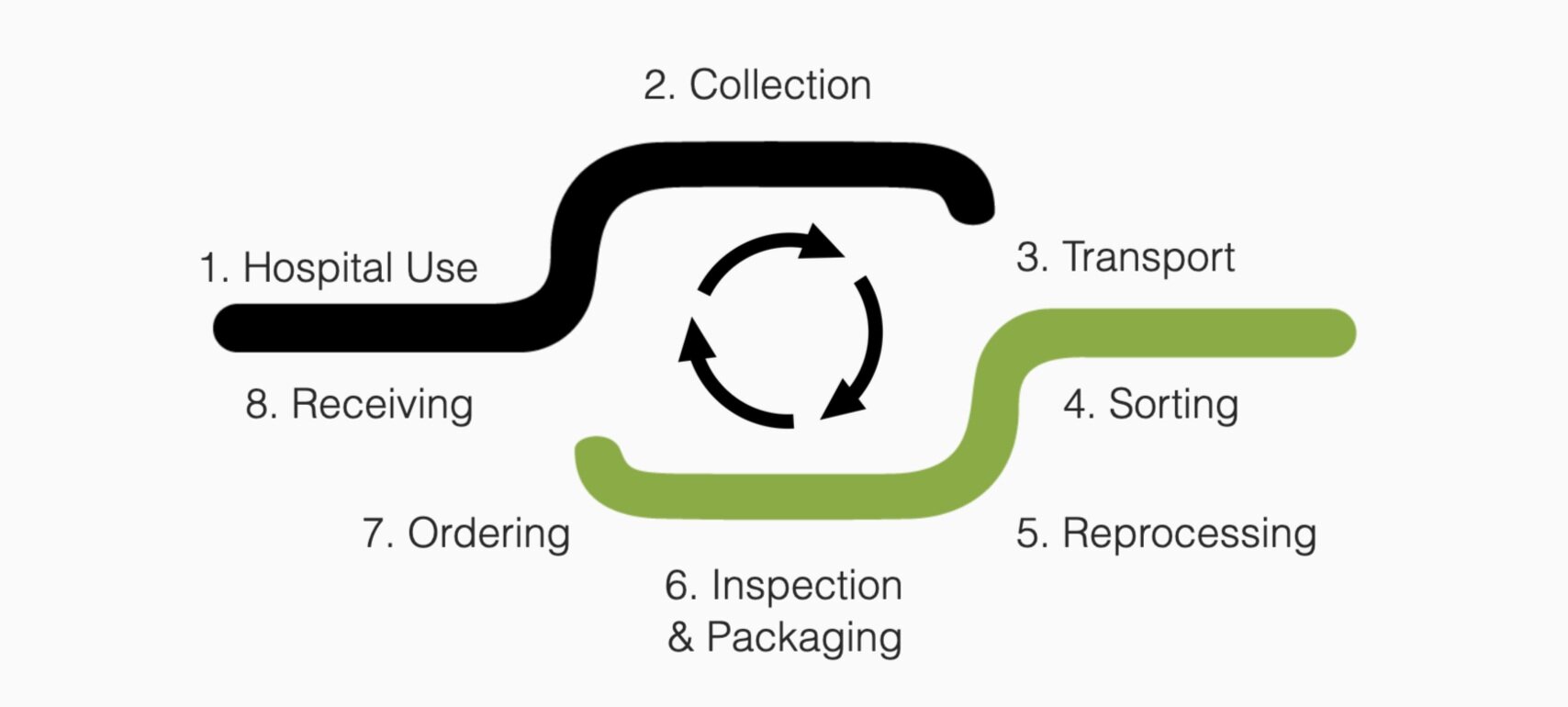
1. Use
Reprocessed devices are stored and used clinically in your hospital in exactly the same manner as new devices. Reprocessed devices are clearly marked so your staff can maximise cost & waste savings.
2. Collection
Devices are collected following use, in a container specific to the device type. Staff notify Medsalv of full containers through an easy to use online portal.
3. Transport
Medsalv co-ordinates the shipping process. Full collection containers are shipped to Medsalv’s facility inside re-usable cartons to minimise cardboard use.
4. Sorting
Used devices are sorted based on: Type and brand of device | Size of device | Visual or functional damage | Collection hospital.
Devices are marked with a Medsalv branded Unique Device Identifier (UDI). This helps Medsalv individually track the device, and clearly brands the device as reprocessed by Medsalv, even when removed from packaging.
5. Remanufacturing
Once the Unique Device Identifier is applied, devices pass through a proprietary cleaning process in Medsalv’s remanufacturing facility. Every device is inspected between 5 and 9 times during remanufacturing, depending on the type of device. Much more than a glorified washing process, remanufacturing by Medsalv includes multiple inspections which ensure devices are returned in safe working order, and will provide an equivalent or better patient experience to new devices.
6. Inspection and Packaging
Every device is subjected to a final inspection prior to packaging, using state of the art testing equipment to ensure functionality and cleanliness. Each device is packaged in a similar manner to the original device, clearly labelled, with clear instructions for use. Labelling makes it easy for clinical staff to distinguish between sizes and shows the device is reprocessed
7. Ordering
Devices are released from quarantine and made available for ordering when all final inspections are complete
8. Returning/Receiving
Devices are returned in clearly marked Medsalv packaging, transported in re-usable cartons. These stackable, re-usable cartons are then used to collect the devices following use. The cartons eliminate the need for wasted cardboard